FDA Warns GLP-1 Patients of Aspiration Risks While Under Anesthesia
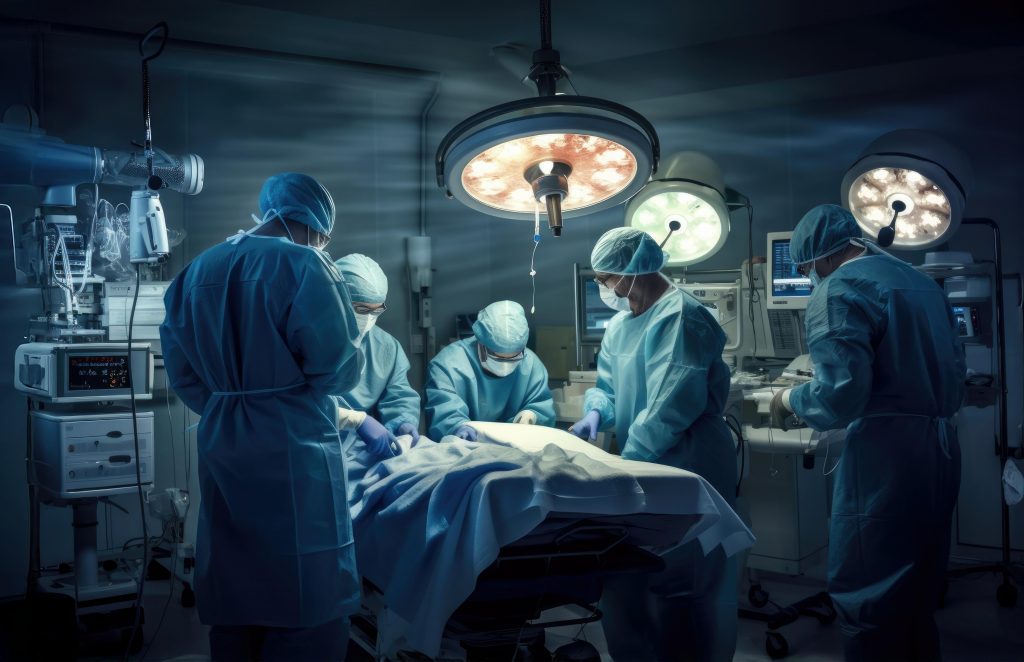
The FDA issued a new warning label for all GLP-1 drugs, alerting patients of rare, potential aspiration risks when undergoing elective surgical procedures.
What Exactly is the Risk?
GLP-1s slow the movement of food through the stomach, creating the “full stomach” feeling that results in significant weight loss. This delayed stomach emptying could put patients at risk of pulmonary aspiration, which is when stomach contents are vomited and enter the airway or lungs while under anesthesia or deep sedation.
This has prompted the FDA warning label on GLP-1 drugs and new recommendations from the American Society of Anesthesiologists for GLP-1 users undergoing surgery.
Updated Recommendations
It is very important to inform your surgeon, anesthesiologist and other healthcare providers of your GLP-1 use. Depending on your risk for pulmonary aspiration, your surgical team may recommend preventive actions like following a liquid-only diet for 24 hours or more before surgery. If you are determined to be at high risk for delayed stomach emptying (e.g., you are in the escalation phase of GLP-1 drugs), your surgery may have to be postponed until the risk decreases.
To see the FDA’s warning label for a specific GLP-1, search the drug’s name at https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/.


 OFFICE LOCATION & HOURS:
OFFICE LOCATION & HOURS:
 MEETING INFORMATION:
MEETING INFORMATION: FAXES:
FAXES: